- Contact Person : Mr. Zheng Haipeng
- Company Name : Yantai Xinghua Biochemical Products Co., Ltd.
- Tel : 86-535-7319623
- Fax : 86-535-7319623
- Address : Shandong,Laiyang,Fushui South Road, Laiyang City, Shandong Province
- Country/Region : China
- Zip : 265200
Sodium Hyaluronate Injection Grade
Introduction:
Hyaluronic acid(HA) is a straight chain macromolecular mucopolysaccharide composed of repeat disaccharide units of glucuronic acid and N-acetylglucosamin. It widely consists in the extracellular space of human and animal tissue, vitreum, umbilical cord, skin joints synovia and cockscomb, etc. The commercial HA is commonly a sodium salt, called sodium hyaluronate, habitually called hyaluronic acid. HA is a new biological at home and abroad. Its molecular weight is from several ten thousand to several million. It's aqueous solution has outstanding moisture keeping ability, high viscoelasticity and lubricity.
Function:
1, Improving joint function
2, Natural moisturizing lubricant
3,It can prevent arteriosclerosis, pulse disorder and the occurrence of the disease such as the brain atrophy.
4.It is widely used in cosmetics 5.moisture retain 6.anti-ageing, lotion,cream
Specification:
a.Food grade b.Cosmetic grade c.Eye drop grade d.Injection grade
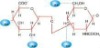
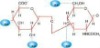
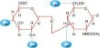
Flow Chart of HA Production
1.Raw materials
2.Sterilization
3.Fermentation
4.Filtration
5. Absorption
6. CPC Isolation
7. Ethanol precipitation
8. Dewatering
9. Drying
10.Packing
Specifications:
| Sodium Hyaluronate( Injection Grade) | |
| Items | Specifications |
| Appearance | White Powder |
| Solubility | 100% soluble in water |
| Odour | Characteristic |
| Taste | Characteristic |
| Identification | Positive |
| Identification of Sodium | Positive |
| Sodium Hyaluronate | 95%-105% |
| Glucuronic Acid | Min.46% |
| Appearance of Solution | Clear Solution |
| Transparency | 99.90% |
| Molecule Weight (Dalton) | ≥3.0 x 106 |
| Intrinsic Viscosity | Min.3.0 m3 /kg |
| Ph | 5.0-8.5 |
| Protein | Max.0.1% |
| Nucleic Acid | Max.0.5% |
| Chlorides | Max.0.5% |
| Loss on drying | Max.10% |
| Residual Isopropanol | Max.0.5% |
| Heavy Metal | Max.10ppm |
| Iron | Max.80ppm |
| Total plate count | Max.10cfu/g |
| E.Coli | Negative |
| Salmonella | Negative |
| Bacterial Endotoxin | Max.0.05IU/mg |
| Sterle Test | Complies |
Sodium Hyaluronate Injection Grade